Borengite
Category: Plutonic
Type Vein rock.
Commons Borengite is very rare vein ultra-potassic rock with the highest recorded K2O concentrations (more than 14 wt.%) among alkalic rocks.
Name origin Rock name comes from the type locality Båräng (Eckermann, 1960).
Locality Båräng, Alnö island, Västernorrland, Sweden.
GPS:
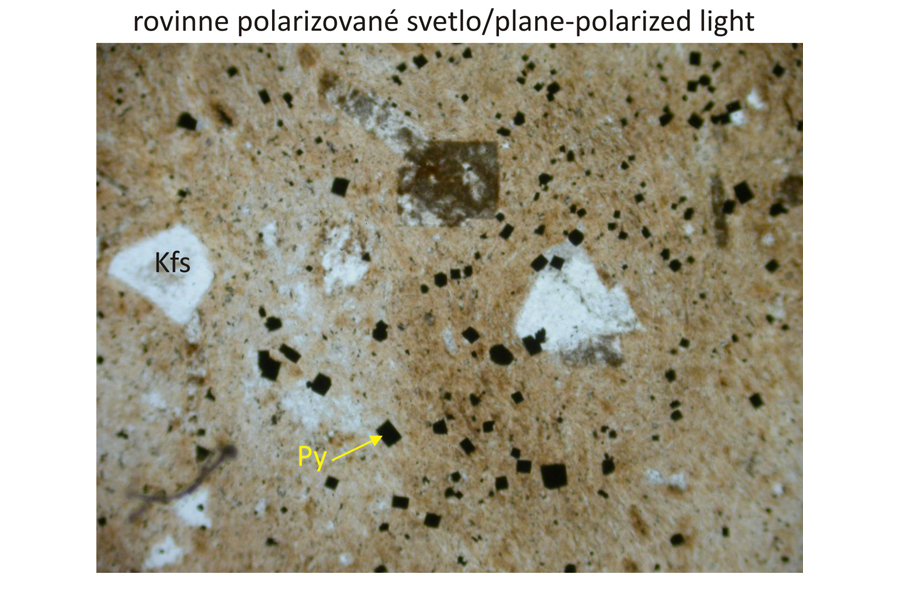
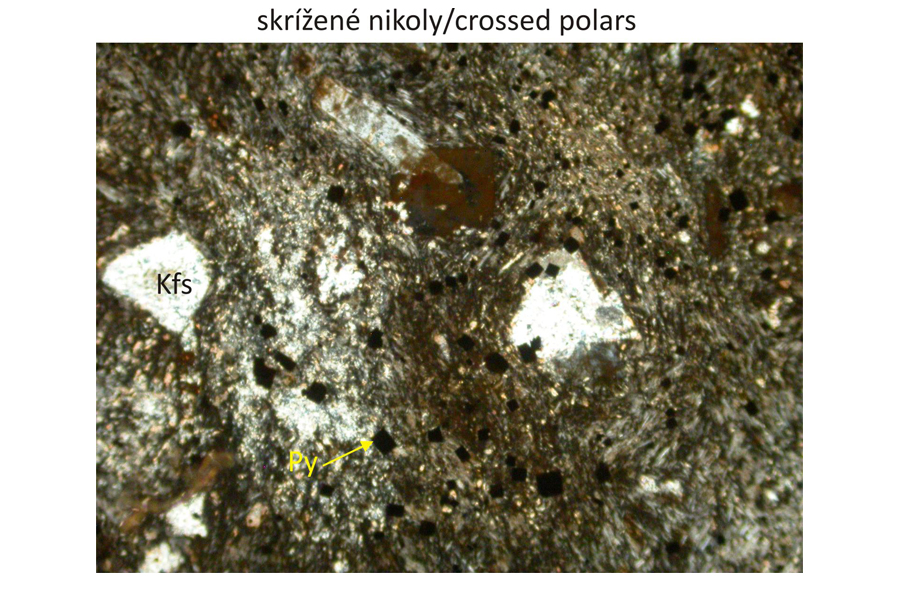
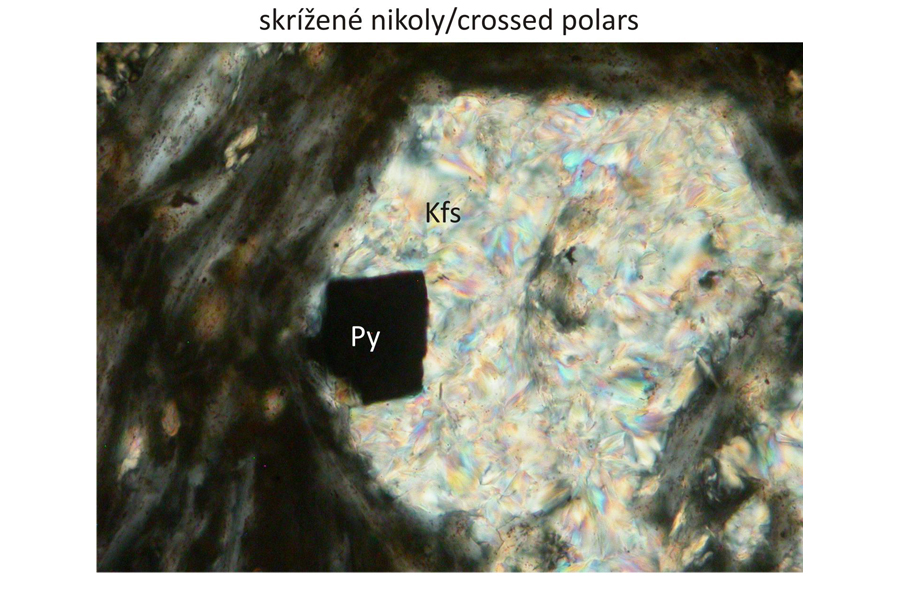
Major minerals K-feldspar (orthoclase), fluorite, sericite.
Accessory minerals Apatite, pyrite.
Classification
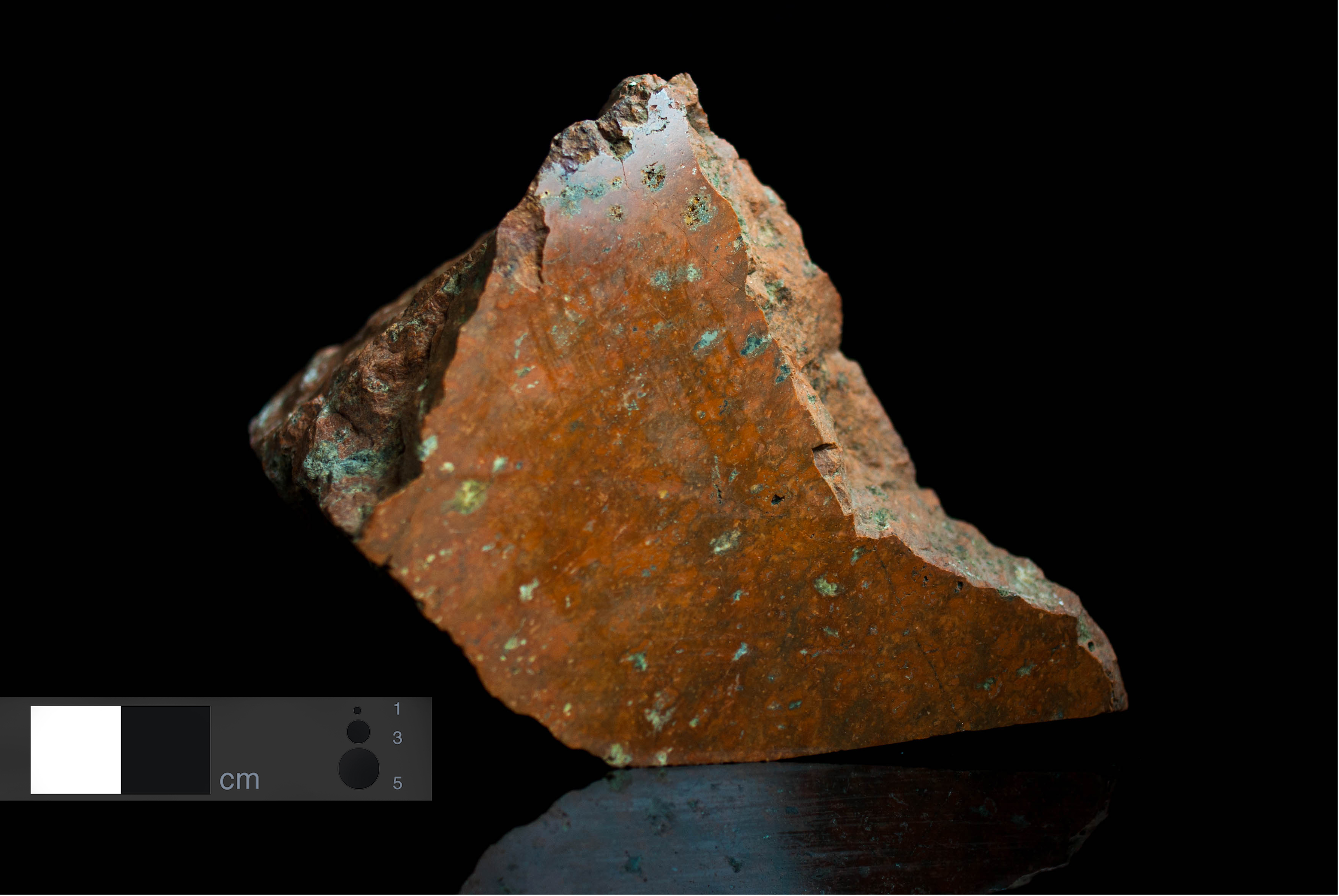
Colour Red-brown, orange to brown-orange.
Structure Aphanitic.
Granularity Fine-grained rock.
Texture
Alterations High degree of sericitization and K-metasomatism.
Petrographic characteristics Fine-grained vein rock of red-brown colour (high K2O contents and very low Na2O)contents. Rare phenocrysts are composed of orthoclase disseminated in trachytic matrix.
Usage Without practical usage.
Literature Eckermann, H. von, 1960: Borengite; a new ultra-potassic rock from Alnoe island. Arkiv foer Mineralogi och Geologi, 36, 519-528. Le Bas, M.J., 1981: Carbonatite magmas. Mineralogical Magazine, 44, 133-140. Mitchell, R. H. & Bergman, S.C., 1991: Petrology of Lamproites. Plenum Press, New York, 447 pp. Foley, S.F., Venturelli, G., Green, D.H. & Toscani, L., 1987: The ultrapotassic rocks: Characteristics, classification, and constraints for petrogenetic models. Earth Sci. Rev., 24, 81-134.
Photomicrographs

Normative composition
Borengite is an ultra-potassic alkalic rock. However, normative foids (e.g. nepheline – ne) or normative alkalic pyroxene (e.g. acmite – ac), typical of alkalic rocks, are missing in their norm. High K2O content is reflected in normative orthoclase - or, and minor Na2O is stored in normative albite - ab. Borengite is Si-saturated rock with normative quartz– q and hypersthene – hy. Peraluminous character is corroborated by normative corundum - c.
Normative minerals
SiO2
TiO2
ZrO2
Al2O3
Fe2O3
FeO
MnO
MgO
CaO
Na2O
K2O
P2O5
F
S
CO2
Total
Molar proportion of normative mineral
Molecular mass of normative mineral
Weight % of normative mineral
Oxide
(wt. %)
56.96
0.26
20.24
1.11
1.18
0.07
0.29
1.93
0.18
14.08
0.06
1.47
97.83
Molecular
weight
60.08
79.88
101.96
159.69
71.85
70.94
40.31
56.08
61.98
94.20
141.95
44.01
Molecular
proportion
0.9481
0.0033
0.1985
0.0070
0.0174
0.0010
0.0072
0.0344
0.0029
0.1495
0.0004
0.0334
ap
0.0014
0.0004
0.0004
328.68
0.14
il
0.0033
0.0033
0.0033
151.75
0.49
cc
0.0330
0.0330
0.0330
100.09
3.30
or
0.8968
0.1495
0.1495
0.1495
556.67
83.21
ab
0.0174
0.0029
0.0029
0.0029
524.46
1.52
c
0.0461
0.0461
101.96
4.70
mt
0.0070
0.0070
0.0070
231.54
1.61
zvyšky
0.0072
0.0072
hy
0.0144
0.0072
0.0072
0.0072
116.17
1.67
q
0.0194
0.0194
60.08
1.17
D: -0.0194
Mg/(Mg+Fe2+): 0.500
Total of normative wt. % 97.83
Comment High CO2 content is stored in normative calcite – cc.
Chemical composition
Borengite is peraluminous alkalic rock with extremely high K2O content and very low Na2O concentration. The K/Na molar ratio > 3 defines borengite as ultrapotassic rock in sense of the classification proposed by Mitchell and Begman (1991). After Foley et al., (1987), borengite should not belong to ultrapotassic rocks owing to a low MgO content. According to this classification, the ultrapotassic rocks are defined as those having K2O > 3 wt. %, the K2O/Na2O ratio > 2 and MgO > 3 wt. %. According to SiO2 content, borengite belongs to intermediate rocks (see chemical analysis).
-
SiO2
56.96TiO2
0.26Al2O3
20.24Fe2O3
1.11FeO
1.18MnO
0.07MgO
0.29CaO
1.93Na2O
0.18K2O
14.08P2O5
0.06H2O+
1.25H2O-
0.36CO2
1.47SO3
0.65F
0.37Total
100.46Mg(Mg/Fe2+)
0.30A/CNK
1.06A/NK
1.30